Prevotellin-2, an antibiotic discovered in the human gut microbiome, has demonstrated anti-infective efficacy on par with polymyxin B, an FDA-approved antibiotic used today to treat multidrug-resistant infections. , suggesting that the human gut microbiome may one day contain antibiotics. Find a clinical application. Credits: Cesar de la Fuente, Ami S. Bhatt
The average human gut contains about 100 trillion microbes, many of which are constantly competing for limited resources. “It’s such a tough environment,” says Cesar de la Fuente, presidential assistant professor of bioengineering and chemical and biomolecular engineering in the School of Engineering and Applied Science, psychiatry and microbiology at the Perelman School of Medicine. and within chemistry. School of Arts and Sciences.
“You have all these bacteria coexisting, but also fighting each other. That kind of environment might promote innovation.”
In this conflict, La Fuente’s lab sees the potential for new antibiotics, which may one day help humanity stockpile its own defenses against drug-resistant bacteria. After all, if the bacteria in the human gut have developed new tools to fight each other to survive, why not use their weapons against them?
In an essay the cellLa Fuente and Amy S. Bhatt, a professor of medicine (hematology) and genetics at Stanford, surveyed the gut microbiomes of nearly 2,000 people and discovered dozens of potential new antibiotics.
“We think of biology as a source of information,” says de la Fuente. “Everything is just code. And if we can come up with algorithms that can sort through that code, we can dramatically speed up antibiotic discovery.”
In recent years, La Fuente’s lab has made headlines for finding antibiotic candidates everywhere, from the genetic information of extinct creatures such as Neanderthals and woolly mammoths to bacterial clusters that the lab uses artificial intelligence. Analyze the genetic material.
“One of our primary goals is to mine the world’s biological data as a source of antibiotics and other useful molecules,” says de la Fuente.
“Instead of relying on traditional, painstaking methods that involve collecting soil or water samples and purifying active compounds, we use the vast array of biological information found in genomes, metagenomes and proteomes. This gives us allowing us to discover new antibiotics at digital speed.”
Given that bacteria grow rapidly, de la Fuente and his co-authors hypothesized that an environment that encourages competition—such as the human gut—might be home to many unknown antimicrobial compounds. “When there’s a scarcity of resources,” de la Fuente points out, “that’s when biology really comes up with innovative solutions.”
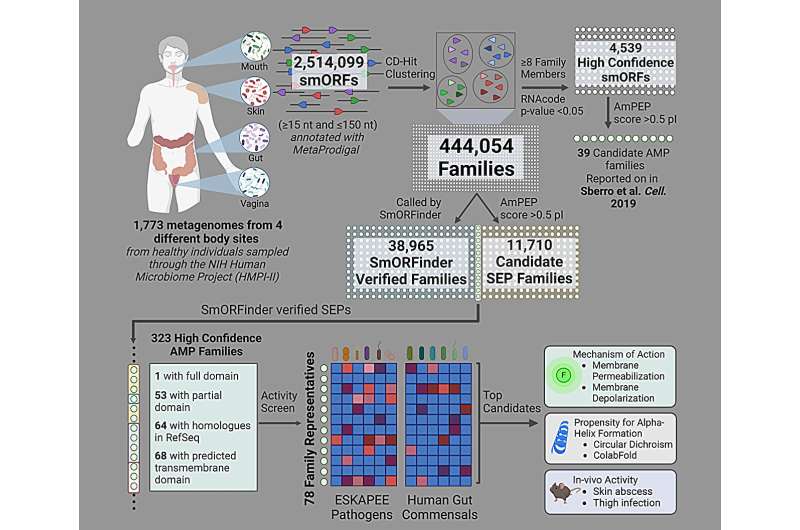
Penn Engineering and Stanford researchers used AI to help identify dozens of potential antibiotic candidates, by analyzing genetic sequences in about 2,000 different human gut microbiomes. Credits: Cesar de la Fuente, Ami S. Bhatt
The group focused on peptides, short chains of amino acids, which have already shown promise as new antibiotics.
“We computationally mined over 400,000 proteins,” says de la Fuente, referring to the process in which an AI reads letters of genetic code and, trained on a set of known antibiotics, predicts Which genetic sequence may have antimicrobial properties?
“Interestingly, these molecules have a different composition than what is traditionally known as antimicrobial,” says Marcelo DT Torres, a research associate at the de la Fuente laboratory and first author of the paper. “The compounds we discovered form a new class, and their unique properties will help us understand and expand the antimicrobial spectrum.”
Of course, these predictions must then be confirmed empirically; After finding several hundred antibiotic candidates, the researchers selected 78 to test against real bacteria.
After synthesizing these peptides, the researchers exposed bacterial cultures to each peptide and waited 20 hours to see which peptides successfully inhibited bacterial growth. In addition, the team later tested the antibiotic candidate in animal models.
More than half of the peptides tested—whether they inhibit the growth of friendly or pathogenic bacteria—and the leading candidate, prevotilin-2, showed anti-infective efficacy on par with polymyxin B, an antibiotic approved by the FDA today. day is used. Treatment of multidrug-resistant infections, suggesting that the human gut microbiome may contain antibiotics that will one day find clinical application.
“Identifying prevotilin-2 as having equivalent activity to one of our last-resort antibiotics, polymyxin B, was quite a surprise to me,” says Bhatt.
“This suggests that mining the human microbiome for new and attractive classes of antimicrobial peptides is a promising avenue for researchers and clinicians, and especially for patients.”
Additional Information:
Mining human microbiomes reveals an untapped source of peptide antibiotics, the cell (2024). DOI: 10.1016/j.cell.2024.07.027. www.cell.com/cell/fulltext/S0092-8674(24)00802-X
Journal Information:
the cell
Provided by the University of Pennsylvania
quote: Mining the Microbiome: Discovering New Antibiotics in the Human Gut (2024, August 19) Retrieved 20 August 2024 from https://phys.org/news/2024-08-microbiome-uncovering-antibiotics- human-gut.html
This document is subject to copyright. Except for any fair dealings for the purpose of personal study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.
#Mining #microbiome #discovering #antibiotics #human #gut